Introduction
Due to the relentless study of scientists, different kinds of unknowns are being known today and new wonders are being revealed. Today, People are starting to think that in this age of science, nothing is really impossible. It seems everything is within our hands. And that is being possible as a result of the ceaseless journey of rigorous researches on materials science. Likewise, graphene is one of the most remarkable discoveries of the 21st century. Graphene is a carbon allotrope consisting of tightly packed carbon atoms arranged in a 2D honeycomb lattice. It is a thin layer of graphite and this laminar material is usually used in pencil lead. Although some scattered attempts on graphene can be traced back to about 1899, two physicists named Andre Geim and Konstantin Novoselov from the University of Manchester, brought a major breakthrough in the field of material science by discovering the new process of graphene production in 2004.
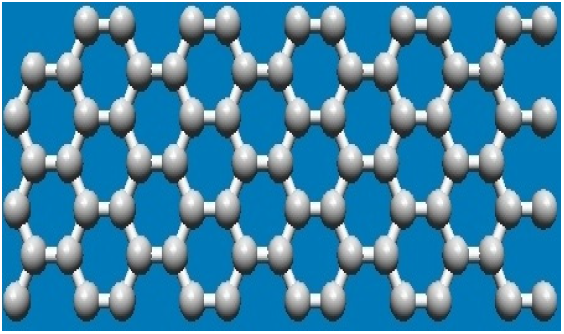 |
| Figure 1: Honeycomb lattice structure of Graphene |
Graphene for its very amazing properties has become the pioneer in modern material science and technology. Graphene has drawn significant attention due to its exceptional electronic, mechanical, thermal and optical properties. And all these properties are derived from the unique electrical band structure of graphene. Still different types of researches on graphene and its properties are being carried out. It has gifted and still gifting us so many dazzling applications in a wide range of fields. It is wise to employ these useful properties of graphene and its derivatives in various functional materials. To date, graphene-based composites have been successfully made with inorganic nanostructures, organic crystals, polymers, metal-organic frameworks (MOFs), biomaterials, and carbon nanotubes (CNTs) and are intensively explored in applications such as batteries, supercapacitors, fuel cells, photovoltaic devices, photocatalysis, sensing platforms. These marvellous graphene properties along with their applications have been tried to be comprehensively presented in this review study.
How to extract graphene?
Graphene has been invented by a playful experiment done by two Russian scientists at the University of Manchester, named Andrei Geim and Kostya Novoselov. The main theme was to make the graphite films ‘as thin as possible’ and investigate their electronic properties. Sticky scotch tape was used to flake off a graphite layer from its body. Scientists did this peeling process repeatedly and finally succeeded to get down to a thin layer of about a few atoms. Although there has been a significant advance in the study of graphene so far, no controlled method of getting crystalline and defects free graphene at large scale has been discovered yet. Many factors of graphene synthesis are under observations. It has been found that high-quality graphene can be synthesized by improving various parameters like temperatures, pressures, cooling rates, catalysts as well as the thickness of the catalysts etc.
Graphene is the miracle material in the 21st century and that is why it could gather unbelievable research interests. As a consequence of rigorous research, various methods are now being developed and used to produce graphene. Some mostly used methods are Mechanical Exfoliation, Epitaxial Growth on Silicon Carbide, Chemical Vapour Deposition, Arc Discharge Method, Reduction of Graphite Oxide, intercalation methods in graphite, unzipping of CNTs, electrochemical and chemical methods, micromechanical cleavage method.
Properties of Graphene
👉Electrical Transport Properties of graphene
Graphene is a 2-Dimensional and 1-Atom thick crystal consisting of sp2 hybridized carbon atoms which are arranged in a hexagonal lattice. Properties like High Charge Carrier Mobility, Ambipolar Field Effect, Anomalous Quantum Hall Effect, Ballistic Transport, Chirality and Weak Antilocation have made the graphene unparallel. Due to the electronic structure graphene has very high charge mobility. Generally, the mobility of graphene lies in between 2000–15000 cm2 v−1s−1. Very recently it has been found that graphene with a clean surface shows carrier mobility up to 200 000 cm2 v−1s−1. Ambipolar field-effect is another crucial graphene property which has instigated its huge applications in electronics. Furthermore, the electronic structure of graphene results anomalous hall effect, chirality and weak antilocation properties. The quantum anomalous Hall effect is an unorthodox demonstration of the topological structure of graphene and can be observed even at room temperature due to the high charge mobility.
👉Optical Properties of graphene
Graphene has very good optical properties like very high optical transmittance, low optical reflectivity, luminescence, photoluminescence quenching properties and fluorescence quenching capability due to its distinctive electronic structures and energy or electron transfer. Interestingly single-layered and bi-layered graphene shows different optical responses; that means graphene shows layer dependent optical transmission properties. It has been found that single-layer graphene has 97.4% optical transmittance and it decreases linearly with the number of layers. Furthermore, luminescence and photoluminescence properties of graphene are successfully being utilized in various biological applications. All these fascinating graphene properties have made it suitably fit for the advanced optical and optoelectronic applications.
👉Mechanical Properties of graphene
Graphene possesses some significant mechanical properties like high in-plane stiffness, highly versatile Young’s modulus and tensile strength due to the hexagonal 2-dimensional lattice consisting of sp2 hybridized C-C bonds. Besides, mechanical properties of graphene are influenced by some other factors like- defects, no. of layers, stitching quality of adjacent boundaries and angle of tilt boundaries. Below table shows a summary of the inherent mechanical properties of different layered graphene.
Table 1: Mechanical Properties of Graphene
👉Thermal Properties of Graphene
Graphene manifests very good heat conveyor properties due to its elastic arrangement of atoms in the honeycomb tight compacted lattice. The thermal conductivity of graphene is about 5 × 103 Wm-1k-1, which is three times as high as diamond and higher than carbon nanotubes (CNT). It has been demonstrated that the specific heat of graphene is bifurcated into atoms vibrations in the lattice (phonons) and free electron contributions. Specific heat, C = Cp + Ce; where Cp = Phonons’ contributions and Ce = free electrons’ contributions. Electrons’ contributions can play a significant role in doped materials, though phonons’ contribution is dominant in graphene generally. Furthermore, the in-plane heat flow direction is always greater than the out-of-plane direction in graphene due to the hexagonal 2-dimensional lattice consisting of sp2 hybridized C-C bonds.
👉Conclusion
The properties of graphene provide basically vast potential for various applications. Graphene and Graphene-based composites have occupied a huge place in advanced engineering applications including energy conversion, energy storage, transparent electrodes, biological and biomedical, etc. Due to the tremendous consumption of limited energy, the earth is forwarding towards future energy crisis and many environmental imbalances. Graphene, for its outstanding electrical conductivity, high specific surface area, and good structural stability, have taken our focus to mitigate this energy and other environmental challenges. Aforementioned review study reveals that graphene is the most promising potential candidate for next-generation materials as well that are being sought immensely for a smooth future. The structure and associated properties of graphene have proved itself as a miracle material indeed.
👉References
[1] K. S. Novoselov, A. K. Geim, S. V Morozov, and D. Jiang, “PGR Tips on writing effective CVs,” Phys. Rev. Lett, vol. 306, no. 52, pp. 666–669, 2004.
[2] J. U. Arikpo and M. U. Onuu, “Graphene Growth and Characterization : Advances, Present Challenges and Prospects,” vol. 8, no. 4, pp. 37–68, 2019.
[3] G. Eda, G. Fanchini, and M. Chhowalla, “Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material,” Nat. Nanotechnol., vol. 3, no. 5, pp. 270–274, 2008.
[4] Q. Wu, Y. Xu, Z. Yao, A. Liu, and G. Shi, “Supercapacitors based on flexible graphene/polyaniline nanofiber composite films,” ACS Nano, vol. 4, no. 4, pp. 1963–1970, 2010.
[5] F. Schedin et al., “Detection of individual gas molecules adsorbed on graphene,” Nat. Mater., vol. 6, no. 9, pp. 652–655, 2007.
[6] C. Lee, X. Wei, J. W. Kysar, and J. Hone, “Measurement of the elastic properties and intrinsic strength of monolayer graphene,” Science (80-. )., vol. 321, no. 5887, pp. 385–388, 2008.
[7] N. Xiao et al., “ACS Nano 2011 Xiao N.pdf,” no. 4, pp. 2749–2755, 2011.
[8] X. Huang, X. Qi, F. Boey, and H. Zhang, “Graphene-based composites,” 2011.
[9] S. Wu, Z. Yin, Q. He, X. Huang, X. Zhou, and H. Zhang, “Electrochemical Deposition of Semiconductor Oxides on Reduced Graphene Oxide-Based Flexible, Transparent, and Conductive Electrodes,” J. Phys. Chem. C, vol. 114, no. 27, pp. 11816–11821, Jul. 2010.
[10] Ryan Muszynski, and Brian Seger, and P. V. Kamat*, “Decorating Graphene Sheets with Gold Nanoparticles,” 2008.
Texpedi.com